©2023 AstraZeneca. All rights reserved. CA-3826 Last updated 07/23
VAXZEVRIA™ is a trademark of AstraZeneca UK Ltd., used under license by
AstraZeneca Canada Inc. The AstraZeneca logo is a registered trademark of
AstraZeneca AB, used under license by AstraZeneca Canada Inc.
VAX2102E
VAXZEVRIA
™
COVID Vaccine
(ChAdOx1-S [recombinant])
INFORMATION FOR THE PUBLIC
2
About this guide
⚫ This guide is for people who are interested in learning more about
the vaccine.
⚫ It only provides information about VAXZEVRIA, known as
ChAdOx1-S (recombinant) COVID-19 Vaccine. It does not
cover other non-ChAdOx1-S (recombinant) COVID-19 vaccines,
COVID-19 treatments or the COVID-19 disease.
⚫ It does not include Health Canada, Public Health Agency of
Canada, provincial or local public health advice.
There is a separate guide wrien for healthcare professionals
available at www.azcovid-19.com.
You should also check the Health Canada Patient Medication
Information section of the Product Monograph available at
www.azcovid-19.com.
What the vaccine is
Geing the vaccine
Precautions
Contents
Benets and
side eects
Who the vaccine is for
How the vaccine
was tested

3
Contents
How to use this guide
⚫ Click on the tabs (above) or the links in Contents
to navigate to each section of this guide
⚫ Click on underlined links to go to content
About this guide 2
What the vaccine is 4
About the vaccine 4
What is in the vaccine 4
More details on what the vaccine is 6
Who the vaccine is for 7
People who should receive the vaccine 7
People who should not receive the vaccine 7
Precautions 8
Important! Tell your doctor or healthcare professional 8
Age, ethnic groups 8
If you are pregnant or breast-feeding 8
Other situations 8
Benets and side eects 10
Benets of the vaccine 10
Possible side eects 11
More details on side eects 13
Geing the vaccine 14
How to get vaccinated 14
What you need to know before you are vaccinated 14
What to expect when geing vaccinated 14
What to do aer you are vaccinated 15
How the vaccine was tested 17
How much testing has been done 17
How the vaccine was authorized 18
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects
4
What the vaccine is
About the vaccine
VAXZEVRIA is a vaccine used to prevent the coronavirus disease
2019 (COVID-19) caused by the SARS-CoV-2 virus. It can be given
to adults 18 years of age and older.
1
It is designed to stimulate your immune system to provide
protection against the coronavirus (SARS-CoV-2). This is the virus
that causes the disease COVID-19, which makes some people very
ill and can even lead to death.
1,12
The vaccine contains a modied common cold virus. The “modied
virus” technology used for this vaccine has already been tested as a
way to make vaccines for other diseases.
17
What is in the vaccine
The vaccine is made up of an active ingredient and other inactive
ingredients to allow the vaccine to be given by injection. The inactive
ingredients also keep the vaccine stable (stop it from changing)
8
, but
there are no preservatives used.
1
The active ingredient is a modied common cold virus, originally
found in chimpanzees. This virus has been altered in the laboratory
so it cannot multiply inside your body.
1
The inactive ingredients are L-Histidine; L-Histidine hydrochloride
monohydrate (both amino acids); magnesium chloride hexahydrate
(supports activities inside cells); polysorbate 80 (a stabilizer);
ethanol (alcohol); sucrose (sugar); sodium chloride (salt); disodium
edetate dihydrate (EDTA, a binding agent); water for injection.
8
The ingredients of this vaccine cannot cause COVID-19 or colds.
1
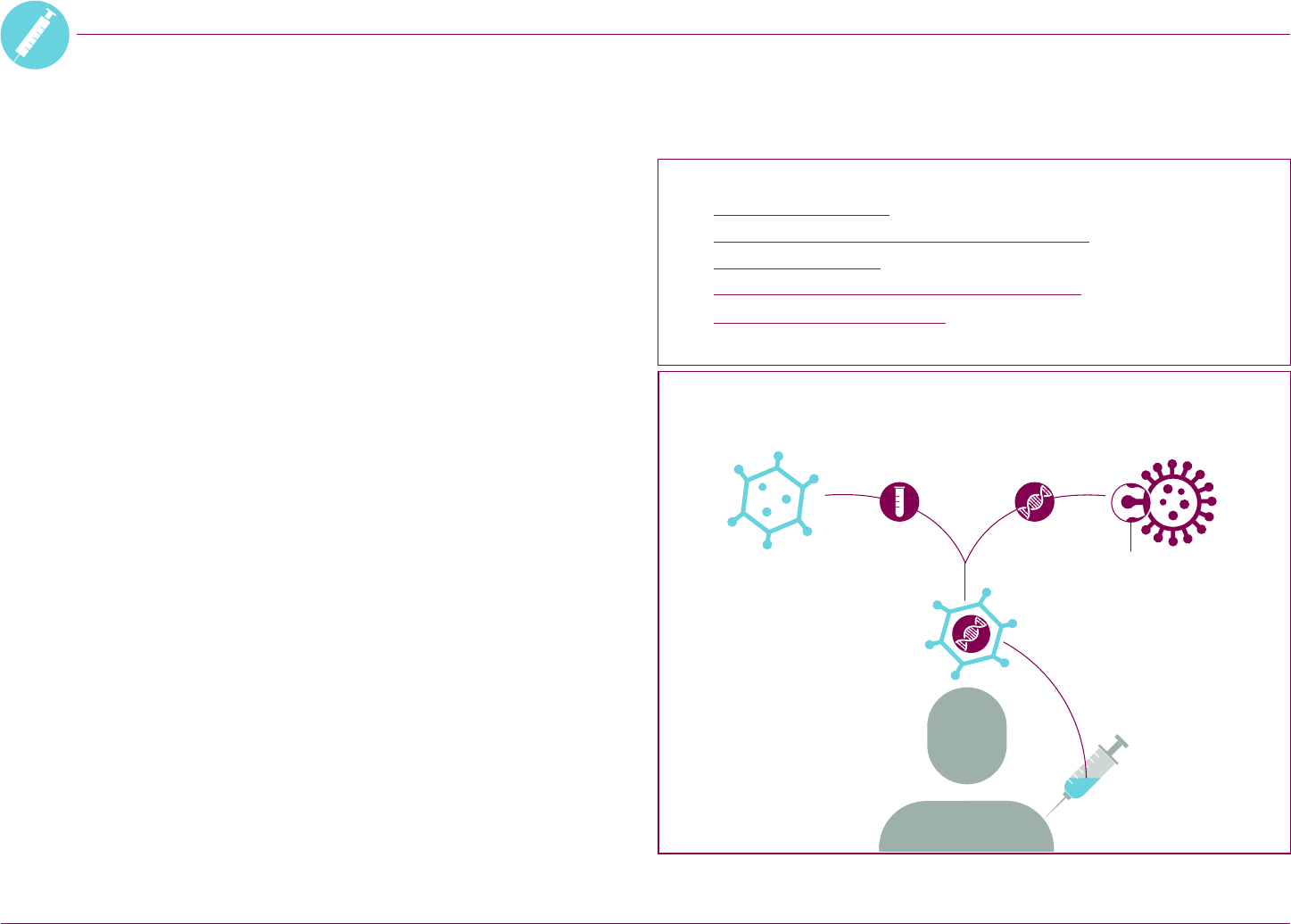
The vaccine contains a
common cold virus, which
is modied so that it can't
multiply in the body.
To make the vaccine,
genetic code for the
coronavirus spike protein
is added to the modied
cold virus.
Spike protein from
coronavirus
Genetic code for
the spike protein
How the vaccine is made
Common cold virus
is modied
Vaccine
Click to see more details on:
▶ How the vaccine works
▶ Does it contain eggs or other animal ingredients?
▶ Does it contain latex?
▶ Does it contain genetically modified organisms?
▶ Information on the ingredients
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

5
More details on what is in the vaccine
Does it contain eggs or other
animal ingredients?
⚫ VAXZEVRIA does not contain milk, lactose, soya, egg,
maize/corn starch, peanuts or gluten.
8
⚫ None of the vaccine ingredients are of human or animal origin,
however the active ingredient of the vaccine is grown using cells
that are of human origin. None of these cells remain aer the
vaccine is puried and is ready to be given.
1,8
⚫ Information on the ingredients is included in the public guide.
Individuals can use this information to decide whether the
vaccine is compliant with their own religious belief systems.
Does it contain latex?
VAXZEVRIA does not contain latex (including the vial
and its stopper).
8,9
Does it contain preservatives?
VAXZEVRIA does not contain any preservatives.
1
Is it a live vaccine?
This vaccine is not like ‘traditional’ live vaccines (which contain
weakened live bacteria or virus) and it does not contain live
coronavirus. The modied common cold virus is live, but it cannot
multiply or spread throughout the body.
5,6,10
If your immune system
does not work properly (immunodeciency) or you are taking
medicines that weaken the immune system (such as high-dose
corticosteroids, immunosuppressants or cancer medicines),
talk to your doctor or healthcare professional before you are
given the vaccine.
1
See How the vaccine works and
The vaccine cannot give you COVID-19 or u.
The carrier virus has been genetically modied in two ways to make
this vaccine:
1,13
⚫ The genetic code needed for the virus to multiply has been removed
and cannot cause the common cold.
⚫ The genetic code for the coronavirus spike protein has been added.
These changes to the common cold virus allow the vaccine to
deliver the spike protein genetic code to your cells without causing
COVID-19.
13
For more information,
see How the vaccine works.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects
6
More details on what is in the vaccine
How the vaccine works
Aer the vaccine is injected, it carries the genetic code for the
spike protein into your body’s cells. Your body starts to produce
the spike protein on its own.
Immune cells in your blood recognize the spike protein as being
an ‘invader’, and this starts a reaction by the immune system.
Your body starts making antibodies and immune cells, called
T-cells, that can target and destroy cells that show the spike
protein. The immune cells also call for more immune cells to be
produced, to help ght the ‘invaders’.
The immune system then goes on to produce memory cells. These
memory cells can spot the coronavirus in the future, by recognizing
the spike protein on the surface of the coronavirus. If the immune
cells do come across the coronavirus in your body, they can call for
more antibodies and T-cells to be produced very quickly. This helps
stop the coronavirus from spreading and helps reduce the damage
caused by the COVID-19 disease.
1,13
Click to see more details on:
▶ Does it contain eggs or other animal ingredients?
▶ Does it contain latex?
▶ Does it contain genetically modified organisms?
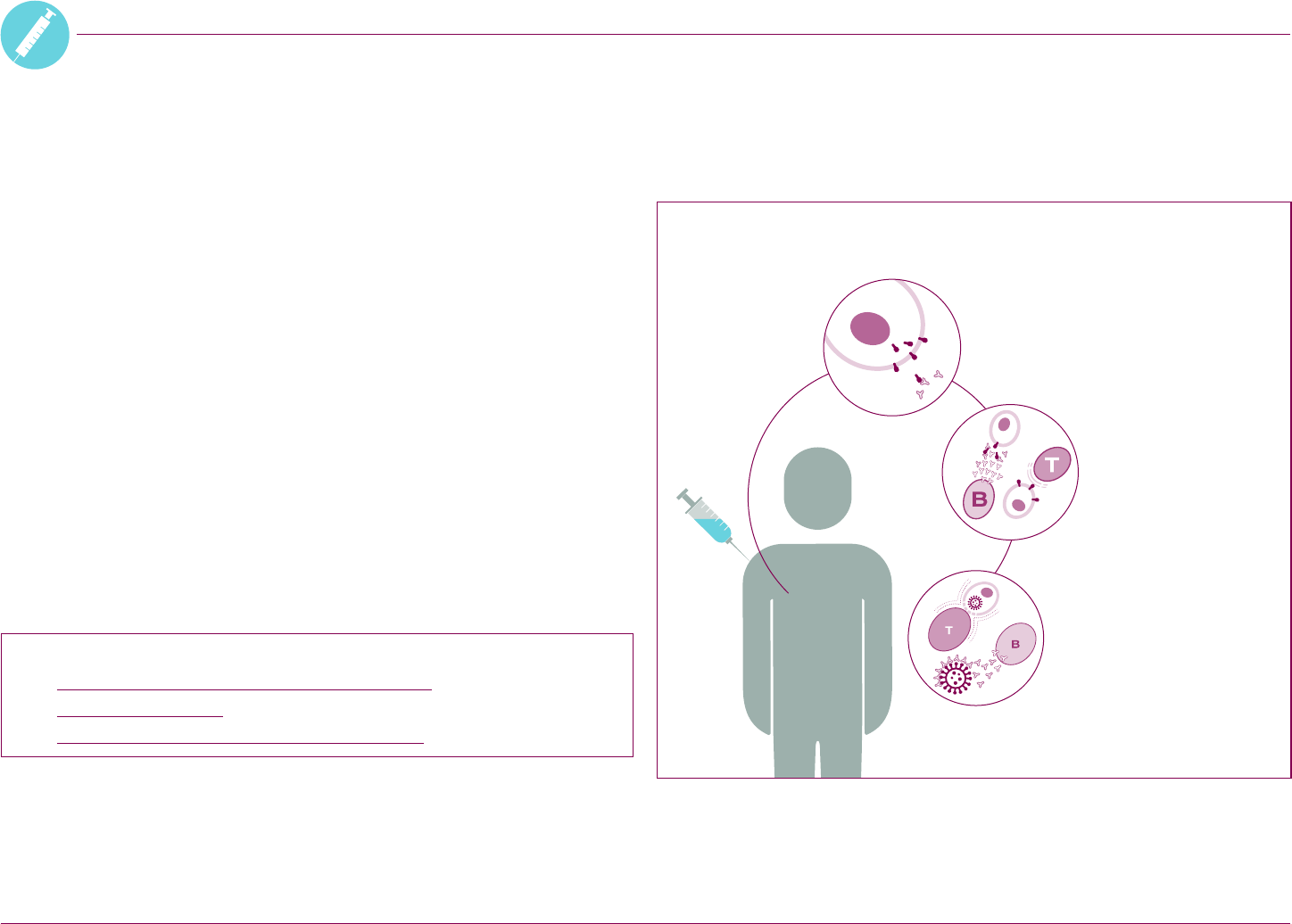
Aer vaccination, your body starts to
produce the spike protein. The body’s
immune cells recognize the spike
protein as an ‘invader’. This starts a
reaction by the immune system.
How the vaccine works in the body
Your body starts
making antibodies
that stick to the spike
protein. It also makes
T-cells that can target
and destroy cells that
show the spike protein.
Your immune system then
produces memory cells.
If they spot the coronavirus
in the future, more
antibodies and T-cells are
produced very quickly.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects
7
Who the vaccine is for
The vaccine is used to protect people aged 18 years
and older against COVID-19.
1
People who have had COVID-19 may be eligible
to receive the vaccine. Speak to your doctor or
healthcare professional if you should or are eligible to
receive the vaccine.
1
You should not receive the vaccine:
1
⚫ If you have ever had a severe allergic reaction to any of the active
or inactive ingredients or a past dose of VAXZEVRIA, listed in
What is in the vaccine.
⚫ Have had a major blood clot occurring at the same time as having low
levels of platelets (thrombocytopenia) aer receiving VAXZEVRIA.
⚫ Have previously experienced episodes of capillary leak syndrome.
⚫ If you have any symptoms that could be due to COVID-19.
⚫ If you are not sure, talk to your doctor or healthcare professional.
There are also important precautions that you should consider and
discuss with your doctor or healthcare professional before you get
your vaccine. See Precautions.
Click to see more details on:
▶ Which ages is it suitable for?
▶ Is it suitable for all ethnic groups?
▶ What if I’m pregnant or breast-feeding?
▶ Will the vaccine affect my ongoing condition?
▶ Can I have it with other vaccines?
▶ Can I have it with other medicines?
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

8
Precautions
Important! Talk to your doctor or healthcare professional:
1
⚫ If you have any allergies or previous problems following administration
of VAXZEVRIA such as an allergic reaction or breathing problems,
or major venous or arterial thrombosis with thrombocytopenia.
⚫ If you have ever had a blood clot or low blood platelets
(thrombocytopenia) in the past or if you have an autoimmune disorder
(illness where the body’s immune system aacks its own cells)
including very low levels of blood platelets.
⚫ If you have ever had
venous sinus thrombosis in the
brain (CVST) with low platelets (thrombocytopenia) or
heparin-induced thrombocytopenia (HIT) or CVST without
thrombocytopenia.
⚫ If you have previously experienced episodes of capillary
leak syndrome.
⚫ If you have ever had a severe allergic reaction aer any other
vaccine injection.
⚫ If you have a weakened immune system due to a medical condition
(immunodeciency) or are on a medicine that aects your immune
system (such as high-dose corticosteroids, immunosuppressants or
cancer medicines).
⚫ If you have any symptoms that could be due to COVID-19, you
should not go out to get vaccinated because you could spread the
infection to others. Talk with your healthcare professional about your
symptoms and geing a COVID-19 test. Your healthcare professional
will advise you when you are able to receive the vaccine.
⚫ If you currently have a severe infection with a high temperature
(over 38°C).
⚫ If you have a problem with bleeding or bruising, or if you are taking a
blood thinning medicine (anticoagulant).
⚫ If you have fainted during or aer previous vaccinations so that you
can be vaccinated lying down.
If you are not sure if any of the above applies to you, talk to your
doctor or healthcare professional before you are given the vaccine.
Which ages is it suitable for?
This vaccine is suitable for adults aged 18 and over.
1
No information is available yet on the use of the vaccine in children
or adolescents younger than 18 years of age.
1
Is it suitable for all ethnic groups?
This vaccine is suitable for all ethnic groups. Clinical trials have been
conducted in UK, US, Brazil, South Africa, Kenya, India and Japan.
There were no restrictions regarding ethnicity.
1
What if I’m pregnant or breast-feeding?
If you are pregnant or breast-feeding, think you may be pregnant,
or are planning to have a baby, talk to your doctor or healthcare
professional before receiving the vaccine. There is limited information
on the use of VAXZEVRIA in pregnant or breast-feeding women. Your
doctor or healthcare professional will discuss with you whether you
can be given the vaccine.
1
A combination of major blood clots and low level of platelets, in some cases together with bleeding, has been
observed very rarely following vaccination with VAXZEVRIA in post-authorization use (see Possible side eects).
1
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

9
Precautions
Will the vaccine aect my ongoing condition?
If you have an underlying health condition, talk to your doctor or
healthcare professional about whether VAXZEVRIA is suitable
for you.
1
Can I have it with other vaccines?
This vaccine hasn’t been tested for use with other vaccines. Talk to
your doctor or healthcare professional if you have recently had or
might have any other vaccines.
1
Ask your healthcare provider how
long to wait aer receiving the ChAdOx1-S (recombinant) COVID-19
Vaccine before you receive another routine vaccine.
Individuals should complete the COVID-19 vaccination course
with VAXZEVRIA.
1
Can I have it with other medicines?
This vaccine hasn’t been tested for use with other medicines.
Talk to your doctor or healthcare professional if you are taking,
have recently taken or might take any other medicines.
1
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

10
Benets and side eects
Benets of the vaccine
1,7,11,14,15,18
Overall benets
VAXZEVRIA is designed to stimulate your immune system to
provide protection against the coronavirus (SARS-CoV-2). This is
the virus that causes the disease COVID-19, which makes some
people very ill and can even lead to death.
How soon protection starts
There may be some protection starting aer the rst dose of
the vaccine, but you will not be optimally protected until aer
receiving the second dose of the vaccine. As with any vaccination,
VAXZEVRIA may not protect everyone who is vaccinated.
Even aer you have had both doses of the vaccine, continue to
follow the recommendations of local public health ocials to
prevent the spread of COVID-19.
How long protection lasts
Because the vaccine is new, there has not been time to conrm
how long protection lasts. As with any vaccine, VAXZEVRIA may
not protect everyone who is vaccinated. It is not yet known how
long people who receive the vaccine will be protected as research
is still ongoing.
Will the vaccine change as the virus mutates?
Scientists will continue to evaluate all new mutations, and any
impact these mutations have on how well the vaccines work.
Click to see more details on:
▶ Will I need further doses in the future?
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

11
Benets and side eects
Possible side eects
1
Like all medicines, this vaccine can cause side eects, although
not everybody gets them. Most side eects are mild to moderate
in nature and resolve within a few days. Fewer side eects were
reported aer the second dose.
Severe allergic reaction (anaphylaxis), severe swelling of the
lips, mouth, throat (which may cause diculty in swallowing or
breathing) have been reported following VAXZEVRIA. Should
you develop any serious symptoms or symptoms that could be an
allergic reaction, seek medical aention right away. Symptoms of
an allergic reaction include:
⚫ hives (bumps on the skin that are oen very itchy)
⚫ feeling faint or light-headed
⚫ changes in your heartbeat
⚫ swelling of your face, lips, tongue or throat
⚫ diculty breathing, shortness of breath or wheezing
Inammation of blood vessels in the skin, oen with a rash and small
red or purple spots (cutaneous vasculitis) has been reported with
unknown frequency.
A combination of major blood clots and low level of platelets, in some
cases together with bleeding, has been observed very rarely following
vaccination with VAXZEVRIA. The majority of the cases occurred
within the rst 3 weeks following vaccination and some cases had a
fatal outcome.
Blood clots in the brain, not associated with low levels of blood platelets,
have been observed very rarely following vaccination with VAXZEVRIA.
The majority of these cases occurred within the rst four weeks
following vaccination. Some cases had a fatal outcome.
Very low levels of blood platelets (immune thrombocytopenia), that can
be associated with bleeding, have also been reported very rarely, usually
within the rst four weeks following vaccination with VAXZEVRIA. Seek
medical aention right away if any of the following symptoms occur
within the rst month following vaccination:
⚫ new severe headaches, worsening or persistent headaches, blurred
vision, confusion or seizures
⚫ shortness of breath, chest pain, leg swelling, leg pain or persistent
abdominal pain
⚫ unusual skin bruising or pinpoint round spots beyond the site
of vaccination
⚫ unexplained bleeding
Very rare cases of capillary leak syndrome (CLS) have been reported
following vaccination with VAXZEVRIA. Some aected patients had a
previous diagnosis of CLS. CLS is a serious, potentially fatal condition
causing uid leakage from small blood vessels (capillaries) resulting
in rapid swelling of the arms and legs, sudden weight gain and feeling
faint (low blood pressure). Seek medical aention right away if you
develop these symptoms in the days following vaccination.
Guillain-Barré syndrome (GBS) is a neurological disorder where
inammation of peripheral nerves causes rapid muscle weakness and
can sometimes lead to paralysis. This has been reported very rarely
aer vaccination with VAXZEVRIA. Seek immediate medical aention
if you develop weakness and paralysis in the extremities that can
progress to the chest and face.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

1212
Benets and side eects
Transverse Myelitis (TM) is a neurological disorder where the
inammation of the spinal cord causes weakness in the arms or
legs, sensory symptoms (such as tingling, numbness, pain or loss
of pain sensation) or problems with bladder or bowel function. This
has been reported very rarely aer vaccination with VAXZEVRIA.
Seek immediate medical aention if you develop weakness, sensory
symptoms or problems with bladder or bowel function.
Aer vaccination, you may have more than one side eect at the same
time (for example, muscle/joint aches, headaches, chills and generally
feeling unwell). If any of your symptoms are persistent, please seek
advice from your healthcare professional.
Side eects that have been reported with VAXZEVRIA were as follows:
Very common (may aect more than 1 in 10 people)
⚫ tenderness, pain, warmth, or itching where the injection is given
⚫ generally feeling unwell
⚫ feeling tired (fatigue)
⚫ chills or feeling feverish
⚫ headache
⚫ feeling sick (nausea)
⚫ joint pain or muscle ache
Common (may aect up to 1 in 10 people)
⚫ swelling or redness where the injection is given
⚫ fever
⚫ being sick (vomiting) or diarrhea
⚫ pain in legs or arms
⚫ u-like symptoms, such as high temperature, sore throat,
runny nose, cough and chills
Uncommon (may aect up to 1 in 100 people):
⚫ sleepiness or feeling dizzy
⚫ decreased appetite
⚫ abdominal pain
⚫ enlarged lymph nodes
⚫ excessive sweating, itchy skin, rash or hives
⚫ sensation like numbness, tingling, pins and needles (paraesthesia)
⚫ reduced sensation of touch (hypoaesthesia)
⚫ ringing in the ears (tinnitus)
If you have any concerns about side eects or your side eects
do not go away, talk to your doctor or healthcare professional.
The vaccine cannot give you COVID-19 or u
Aer vaccination, you may experience u-like symptoms such as
feeling tired, muscle/joint aches, headache, chills or fever. These
side eects are common, and do not necessarily mean you have
the u or COVID-19.
None of the ingredients in this vaccine can cause COVID-19, u or
a common cold. See How the vaccine works for more details.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects
13
More details on side eects
Is there anything I can do to reduce side eects?
⚫ You can take medicines containing acetaminophen or ibuprofen
if you need relief from side eects such as pain and/or fever.
1
⚫ Talk to your doctor or healthcare professional if you have any
further concerns.
Where can I report side eects?
You can report side eects directly as follows:
Are there any long-term eects?
As this vaccine is new, long-term data is not yet available. However,
VAXZEVRIA has been given to thousands of people in clinical
trials. They are being carefully monitored and will be followed up
for 12months. See also How long protection lasts.
Will it aect my fertility?
There is currently no information available on fertility in humans.
Further clinical trials are planned and relevant information will be
provided to your doctor or healthcare professional when available.
1
Reporting Suspected Side Eects for Vaccines
For the general public: Should you experience a side effect following
immunization, please report it to your healthcare professional.
Should you require information related to the management of the side effect,
please contact your healthcare professional. The Public Health Agency of
Canada, Health Canada and AstraZeneca Canada Inc. cannot provide
medical advice.
For healthcare professionals: If a patient experiences a side effect following
immunization, please complete the Adverse Events Following Immunization
(AEFI) Form appropriate for your province/territory (https://www.canada.
ca/en/public-health/services/immunization/reporting-adverse-events-
following-immunization/form.html) and send it to your local Health Unit.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects
14
Geing the vaccine
How to get vaccinated
Vaccination will be oered in dierent ways, to dierent people, in
dierent regions of the country. Check your local arrangement with
a doctor or healthcare professional.
What you need to know before
you are vaccinated
Important! Before you get vaccinated, please read the section
called Precautions.
Scheduling other vaccinations (e.g., the u vaccine)
The use of VAXZEVRIA with other vaccines, including the u
vaccine, has not yet been assessed. Talk to your doctor or
healthcare professional rst if you have recently had or might
have any other vaccinations.
1
No known eect on driving
VAXZEVRIA has no known eect on the ability to drive and use
machines. However, side eects may impact your ability to drive
and use machines. If you feel unwell, do not drive or use machines
1
– see Benets and side eects.
Food, drink and the vaccine
There are no known dietary eects or cautions when you
have this vaccination. If you have a restricted diet, see also
What is in the vaccine.
Geing vaccinated if you are unwell
Talk to your doctor or healthcare professional before your
vaccination if you are unwell, especially if you currently have a
severe infection with a high temperature (over 38° C ) including any
symptoms associated with COVID-19. It is not recommended to
vaccinate people with COVID-19 who are still symptomatic. Your
vaccination may need to be postponed.
1
If you are unwell, you
should not go into the clinic so that you don’t spread it to others.
If you have a long-term illness, speak to your doctor or healthcare
professional about whether VAXZEVRIA is suitable for you. See
also Who the vaccine is for.
Geing vaccinated if you had previous
COVID-19 infection
Individuals with previous COVID-19 infection can be vaccinated
with VAXZEVRIA once they are no longer infectious.
1
What to expect when geing vaccinated
1
How is the vaccine given?
This vaccine is injected by a healthcare professional
into a muscle usually in your upper arm.
Aer receiving the vaccine, your healthcare
professional will observe you for at least
15 minutes for any possible severe allergic
reaction (anaphylaxis).
How many doses will I need?
You will receive 2 injections. The second injection can be given
between 4 and 12 weeks aer the rst. Individuals should complete
the vaccination course with VAXZEVRIA.
1
2 doses
given 4-12
weeks
apart
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

15
Geing the vaccine
You will be told when you need to return for your second injection
of VAXZEVRIA. When VAXZEVRIA is given for the rst injection,
VAXZEVRIA (and not another vaccine against COVID-19) should
be given for the second injection to complete the vaccination
course. It is very important that you return for the second injection,
or the vaccine may not work as well. Individuals should complete the
vaccination course with VAXZEVRIA.
1
Speak to your doctor or healthcare professional if you need
exibility around the dosing schedule.
What do I do if I miss an injection?
If you forget to go back at the scheduled time, ask your doctor
or healthcare professional for advice on how to reschedule. It is
important that you return for your second injection of VAXZEVRIA.
1
Will I need further doses in the future?
Two injections, between 4 and 12 weeks apart, is the currently
recommended schedule.
1
Your doctor or healthcare professional
will tell you what local guidance recommends and when to return for
your second dose.
It is not yet known how long people who receive the vaccine will be
protected for. Studies are planned to examine the long-term eects
of the vaccine. See How long protection lasts.
What to do aer you are vaccinated
Continue to follow local guidance
Follow local rules on masks, handwashing and physical distancing,
before and aer vaccination, and ask your doctor or healthcare
professional for advice.
As with any vaccine, VAXZEVRIA may not protect everyone who
is vaccinated.
Click to see more details on:
▶ How long should I wait to travel?
▶ Will the vaccination affect a COVID-19 test?
Your second dose
1
Your doctor or healthcare professional will tell you when you need
to return for your second injection of VAXZEVRIA, which may be
between 4 and 12 weeks aer the rst injection.
If you forget to go back at the scheduled time, ask your doctor or
healthcare professional for advice. It is important that you return
for your second injection of VAXZEVRIA.
Click to see more details on:
▶ I was given VAXZEVRIA for my rst dose
– can I get a dierent vaccine for my second dose?
Make sure you get your lot number and keep it safe
The person giving you the injection will give you an immunization
record with the name of the vaccine, batch/lot number, and date
of vaccination for the VAXZEVRIA you receive. You need to keep
it safe. This can help to trace the manufacturing details if they are
never needed.
Learn more about COVID-19
Please refer to your national or local health authorities or get more
information from the World Health Organization.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

16
Geing the vaccine
What to do aer you are vaccinated
How long should I wait to travel?
Follow local and international guidance.
Will the vaccination aect a COVID-19 test?
There are two types of tests.
4
⚫ Having the vaccine will not lead to a positive PCR (polymerase
chain reaction) test for COVID-19. The vaccine does not contain
live coronavirus or the part of the virus this test looks for.
⚫ If you are receiving an antibody test, the antibodies produced aer
vaccination may aect the result. This only applies if the test looks
for antibodies against the spike protein of the coronavirus.
I was given VAXZEVRIA for my rst dose – can I get a
dierent vaccine for my second?
You should complete the vaccination course with VAXZEVRIA,
and not another vaccine against COVID-19.
1
Your healthcare professional will discuss this with you, and when
you should get your second dose.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects
17
How the vaccine was tested
Development of VAXZEVRIA has been fast, not rushed. The testing
of coronavirus vaccines has been faster than usual because the
pandemic is a health emergency. No risks have been taken with
vaccine safety.
Regulatory agencies that authorize medicines have clear and strict rules
for the authorization of any new medicine.
How much testing has been done
Worldwide, over 55,000 people have taken part
in testing this vaccine as clinical trial participants,
receiving either vaccine or a placebo.
2
The “modied virus” technology used for
this vaccine has already been tested and
successfully used as a way to make vaccines
for other diseases.
16,17
Employing more
sta and using more
test sites than usual
Running clinical
trials to overlap each
other, instead of one
aer another
Faster and more
ecient recruitment
of test volunteers
Manufacturing
large amounts of
vaccine in advance,
to be ready at once
if regulatory agencies
authorize its use
How the development of VAXZEVRIA has been accelerated
More than
55,000
people
have taken part
in clinical trials
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects

18
How the vaccine was authorized
VAXZEVRIA was authorized for use in relation to the COVID-19
pandemic with terms and conditions. For more information, refer
to the Authorization Terms and Conditions for VAXZEVRIA.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
How the vaccine
was tested
Benets and
side eects
19
References
1. VAXZEVRIA Product Monograph.
AstraZeneca Canada Inc. December 14, 2022.
2. AstraZeneca Pharmaceuticals LP. AZD1222 vaccine met primary
ecacy endpoint in preventing COVID-19 [press release]. hps://www.
astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html.
Published November 20, 2020. Accessed November 20, 2020.
3. AstraZeneca Pharmaceuticals LP. Innovating production and manufacture
to meet the challenge of COVID-19. hps://www.astrazeneca.com/
what-science-can-do/topics/technologies/innovating-production-
and-manufacture-to-meet-the-challenge-of-covid-19.html. Accessed
November 13, 2020.
4. Centers for Disease Control and Prevention. Facts about vaccination
[online] hps://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-
benets/facts.html. Published December 20, 2020. Accessed December
23, 2020.
5. Coughlan L, Mullarkey C, Gilbert S. Adenoviral vectors as novel vaccines
for inuenza. J Pharm Pharmacol. 2015;67:382–399.
6. Dicks MD, Spencer AJ, Edwards NJ, et al. A novel chimpanzee adenovirus
vector with low human seroprevalence: improved systems for vector
derivation and comparative immunogenicity. PLoS One. 2012. hps://doi.
org/10.1371/journal.pone.0040385. Accessed November 20, 2020.
7. Folegai PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the
ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of
a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:
467-78. Accessed December 23, 2020.
8. In House Data, AstraZeneca Pharmaceuticals LP. AZD1222 Allergen
Information Sheet. August 31, 2020.
9. In House Data, AstraZeneca Pharmaceuticals LP. Chemistry,
Manufacturing and Controls Email communication.
November 05, 2020.
10. Morris S, Sebastian S, Spencer A, Gilbert S. Simian adenoviruses as
vaccine vectors. Future Virol. 2016;11(9):649–659.
11. Ramasamy MN, Minassian AM, Ewer KJ. Safety and immunogenicity of
ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young
and old adults (COV002): a single-blind, randomised, controlled, phase
2/3 trial. Lancet 2020;396:1979–93.
12. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2.
Proc Natl Acad Sci. U.S.A. 2020;117:11727–11734.
13. University of Oxford. About the Oxford COVID-19 vaccine. hps://www.
research.ox.ac.uk/Article/2020-07-19-the-oxford-covid-19-vaccine.
Accessed December 23, 2020.
14. University of Oxford. A Study of a Candidate COVID-19 Vaccine
(COV003). ClinicalTrials.gov website. hps://clinicaltrials.gov/ct2/show/
NCT04536051?term=chadox1+ncov19&draw=2&rank=2. Accessed
December 10, 2020.
15. University of Oxford. Investigating a Vaccine Against COVID-19
(COV002) Clinical trials.gov website. hps://clinicaltrials.gov/ct2/show/
NCT04400838. Accessed December 10, 2020.
16. University of Witwatersrand. COVID-19 vaccine (ChAdOx1 nCoV-19) trial
in South African adults with and without HIV-infection. ClinicalTrials.gov
website. hps://clinicaltrials.gov/ct2/show/NCT04444674. Accessed
December 10, 2020.
17. Vemula S and Mial S. Production of adenovirus vectors and their use
as a delivery system for inuenza vaccines. Expert Opin Biol Ther. 2010
October;10(10):1469–1487.
18. Voysey M, Costa Clemens SA, Madhi SA, et al. Safety and ecacy of the
ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim
analysis of four randomised controlled trials in Brazil, South Africa, and
the UK. Lancet. 2020 Dec 8. Online ahead of print.
What the vaccine is
Geing the vaccine
Precautions
Contents
Who the vaccine is for
Benets and
side eects
How the vaccine
was tested
